Are Clinical Trials Still Being “Hidden”?
Research in the USA is confirming what studies in the British Medical Journal have already pointed out. Clinical trials, once completed, are not coming to light for months – even years. And so neither health professionals nor patients are getting the whole story.
Studies that we talked about in 2012 found that many studies were not published even four years after they were completed. Read the full story at: The Case of the Missing Studies: Patients missing critical information.
But researchers from the California Pacific Medical Center Research Institute and University of Rochester School of Medicine and Dentistry set out to discover if this is true of migraine trials in particular. Their findings are disturbing.
The short answer is that yes, the situation is the same with migraine trials. Of 163 trials that were searched, only 57% were available in peer-reviewed literature after 12 months. Searching other literature brought the number up to 70%. Where are the other 30%?
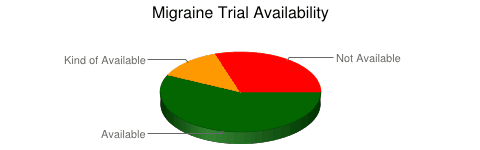
Now it appears that migraine trials have a better record than studies for some other diseases. However, various studies that have investigated this phenomenon have different numbers because of different methods.
The bottom line is this: If you’re looking for migraine or headache trials in the typical places, a huge percentage of trials won’t be there.
This particular study did note that sponsored studies, for example studies sponsored by a big drug company, were actually more likely to be published. Although this doesn’t completely let drug companies off the hook, it does suggest that organization, finances, and public scrutiny, may help to get a trial from start to published.
What kinds of trials are missing? Two kinds in particular, according to the study’s authors.
First, small trials. Trials with fewer than 141 subjects were less likely to be promptly published. Typically, these trials are less valuable than larger studies. But they are also easier to do, and often very well designed. So in some cases these studies are actually more useful than larger studies.
Second, trials with negative results. This is a well known problem. Maybe there are 10 trials on a certain migraine medication. Four show positive results, six no results at all, or negative results. What if three of the six were never published? It sure gives us a different picture, doesn’t it?
Add this to the fact that drug companies are naturally going to promote more positive studies, and all of a sudden you have a “miracle pill” that should take care of almost any migraine! Except that the studies really aren’t saying that.
This is a very challenging problem to solve. To have well-designed, well-reported, honest trials, you need oversight and accountability. And this means world-wide oversight and accountability. But there are biases and politics in every country, and even world-wide.
Medical research has come a long way, and international cooperation is allowing us to learn more than ever before. Unfortunately, in order to make good research happen, you still need education, honesty, and money. Even then, human researchers are not perfect.
The authors of this study, who have studied this area for a long time, make some suggestions about how the system can be improved.
Meanwhile, doctors and patients need to be aware that studies are a tool, not an infallible standard. Treatments which are studied (whether drug or non-drug) are likely not as great as they may seem.
To read a summary of this study, see How transparent are migraine clinical trials? For the full report, see the article at the journal Neurology.

MigraineRF
10 September 2014 @ 6:01 am
A new piece on the problem of missing published research. MRF posts the results of EVERY migraine research… http://t.co/wH3Fs2b0QH
TDHblog
12 September 2014 @ 2:05 pm
30% of clinical trial findings on #migraine go unpublished, often ones with negative results by @migraine_blog. http://t.co/RKdsDE7aNp