Big News: First CGRP Inhibitor for migraine Approved in US – Aimovig
Yesterday, the FDA approved the first of a new class of migraine drugs – CGRP monoclonal antibodies. We’ve been talking about these drugs – and this one in particular, for many years. And it should be available in the USA in a couple of weeks.
 The drug is being sold as Aimovig, but we’ve known it by its earlier names, AMG 334 and erenumab.
The drug is being sold as Aimovig, but we’ve known it by its earlier names, AMG 334 and erenumab.
Aimovig, and it has been approved as a migraine preventative. This is what the FDA had to say about it yesterday:
The U.S. Food and Drug Administration today approved Aimovig (erenumab-aooe) for the preventive treatment of migraine in adults. The treatment is given by once-monthly self-injections. Aimovig is the first FDA-approved preventive migraine treatment in a new class of drugs that work by blocking the activity of calcitonin gene-related peptide, a molecule that is involved in migraine attacks.
[FDA Press Release]
For a somewhat lighthearted basic explanation from 2012, check out The Secret of CGRP.
Aimovig dosage
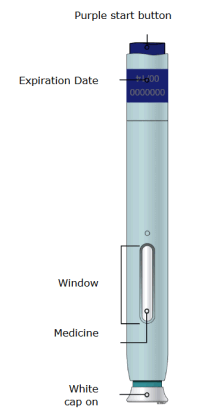 Aimovig is currently a 70mg injection taken each month. Some patients may take a double dose, which would mean two injections of 70mg taken the same day. The injection, done with a pen-like “autoinjector”, is taken in the abdomen, upper arm, or thigh. It’s designed to be injected by the patients, but there are some things to watch for, so talk to your doctor. The injection takes about 15 seconds.
Aimovig is currently a 70mg injection taken each month. Some patients may take a double dose, which would mean two injections of 70mg taken the same day. The injection, done with a pen-like “autoinjector”, is taken in the abdomen, upper arm, or thigh. It’s designed to be injected by the patients, but there are some things to watch for, so talk to your doctor. The injection takes about 15 seconds.
Aimovig Side Effects and Other Concerns
Some of the headlines are claiming that this is a wonderful new side-effect-free medication, which is not strictly true. The side effects do seem to be minimal, which is certainly the general advantage. However, a few patients were irritated by the injection itself. Some also experienced constipation. There are also cautions because of the unknowns of taking Aimovig during pregnancy, and researchers are concerned that there may be an increased risk of preeclampsia.
But for most people, the side effects should be minimal or non-existent.
Advantages of Aimovig
Aimovig seems to be helpful for patients with episodic and chronic migraine, although you will need to discuss with your doctor if your attacks are frequent enough to warrant this type of medication.
As mentioned before, you may experience fewer (or no) side effects with this medication compared to what you have tried before. If you have not used a preventative medication before, your doctor will probably try some other drugs or treatments first before Aimovig.
Aimovig may not prevent all your attacks. If they cut your attacks by a third or a half, that will be a significant improvement.
However, using Aimovig with other treatments could mean a much more significant improvement. And remember, this is only the first of this type of drug to hit the market – other CGRP drugs could work better for you. And there are a whole new class of drugs coming after that.
Another interesting note – this drug is currently being investigated as a possible treatment for hot flashes during menopause.
We will look forward to hearing about your experiences with Aimovig. Once you’ve tried it, come back and comment!

19 May 2018 @ 1:34 am
Please could you tell me if this treatment is going to be available in the UK and when. Thank you
19 May 2018 @ 7:50 am
Thanks for posting this review of the new medicine.
Does anyone have information on its cost? I have heard quoted an outrageous price , but I don’t know how true it is ($6,000 yearly, $500/shot??). We can hope that insurance covers most of the cost, and soon.
21 May 2018 @ 8:37 am
This would be wonderful if it reduces aura. Has it been proven to help auras? Thanks
25 June 2018 @ 9:59 am
Can anyone comment on how long it’s taking to hear from the company about receiving the medicine? My dr office sent in the paperwork nearly a month ago, stating I should hear from the drug company in a couple weeks. I’ve never heard from them. Dr confirmed they acknowledged receipt of my info.
Any one else waiting???
29 June 2018 @ 12:23 pm
My neurologist said that the company says they did not anticipate the demand, and are waiting for a sufficient supply of the medicine. I’m sure it has something to do with the first 2 shots of very expensive medicine being initially free. The experts think we’ll be able to judge after 2 shots/ 2 months if there’s a reduction or “cure” to warrant the cost of estimated $550-$600 per shot monthly. I’m curious to see what Medicare RX plans will do for coverage. If at Tier 4, it’s still lots of money for the average income person, especially since the insurance companies provide the pain meds freely and cheaply.
21 August 2018 @ 1:31 pm
My husband has chronic migraine and his neurologist prescribed Aimovig for him. The first shot was just delivered today (so he hasn’t tried it yet). I can report that it looks like the cost per dose is around $575, but his insurance picked up the entire cost, except a $20 co-pay. For reference, he has Medicare (Senior Advantage Kaiser). Hopefully that helps with the Medicare question. That might not be true for all, but I certainly was relieved that it was only $20.