New Device Approved by FDA to Fight Cluster Headache (video)
Last month the announcement was made that gammaCore, a non-invasive vagus nerve stimulation (nVNS), was approved by the FDA for the treatment of episodic cluster headache.
We’ve been watching the development of gammaCore for the last few years. It’s already been approved in quite a few countries around the world, but this is the first approval in the United States.
This device has shown promise not only in the treatment of cluster headache, but also migraine. Although this recent approval was for episodic cluster, there is some evidence that vagus nerve stimulation may help patients with chronic cluster and chronic migraine.
This particular device is used directly by patients, and is hand-held – small enough to carry along. It’s non-invasive and painless – simply held against your neck for 90 seconds.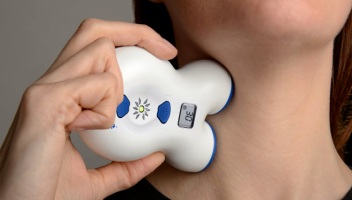
At the time of writing this post, this device is not yet available in the United States. However, you can check out the gammaCore US website for updates.
Meanwhile, here is a video from a chronic cluster headache sufferer (he also suffers from SUNCT), explaining how he has found gammaCore helpful.
