GammaCore Device Approved for Migraine Prevention
The GammaCore device was approved late last month by the FDA in the USA for migraine prevention. It was already approved for preventative and acute treatment for cluster headache, and acute (as-needed) treatment for migraine.
GammaCore is a vagus nerve stimulator, a device which is non-invasive and drug free. The recommended treatment is three times a day, with two two-minute treatment each, as explained in this graphic from the official site:
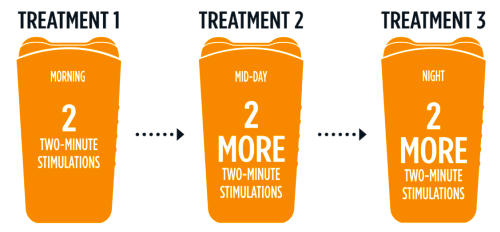
Nerve stimulators have been available for a long time, but often at a very high price, and they are often implanted surgically.
GammaCore is a small device that you can carry with you, to either use at the onset of migraine pain, or to use daily as a preventative. It’s simply placed against the neck when you need a treatment.
Side effects from this device seem to be rare and mild, and clinical trials have shown a lot of promise for quick and lasting pain relief. If you’re interested in a drug-free option, there is a lot of information on the official site of GammaCore here.
